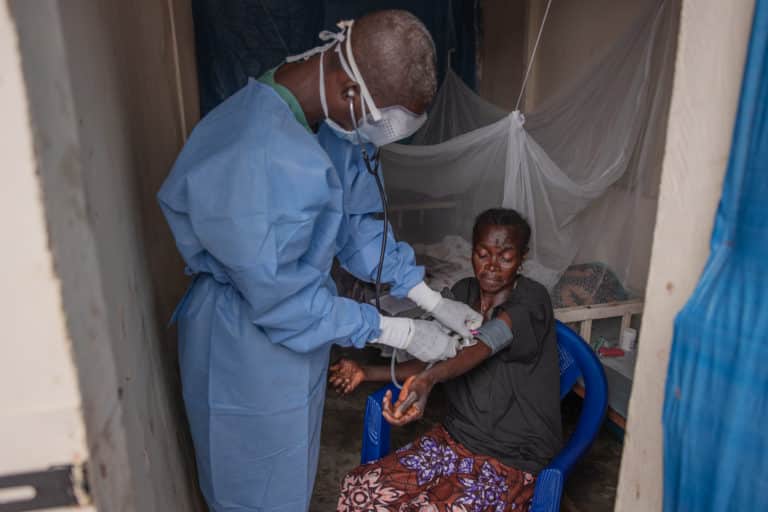
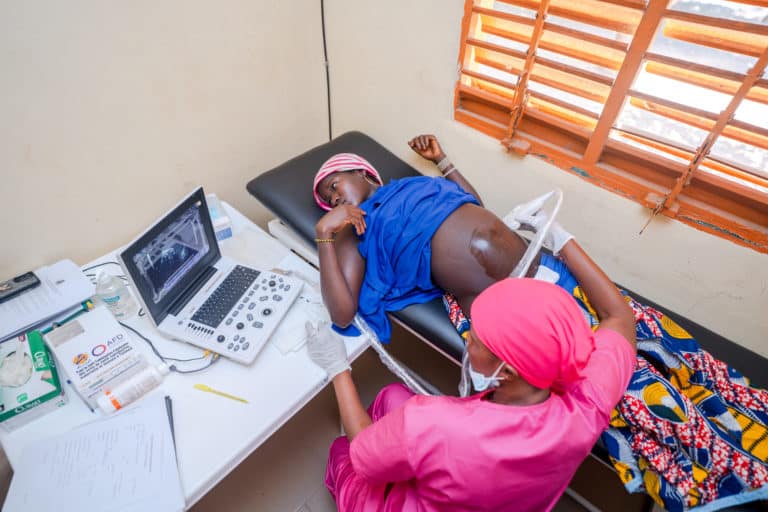
As Ebola outbreaks become increasingly common in several countries across sub-Saharan Africa, identifying effective vaccination strategies against the disease can help save countless lives. The clinical trial – conducted by the PREVAC (Partnership for Research on Ebola VACcination) international consortium comprising major research institutes such as the French National Institute of Health and Medical Research (Inserm), the London School of Hygiene & Tropical Medicine and the U.S. National Institutes of Health (NIH) – aimed to assess three different vaccine strategies to prevent Ebola. The trial results confirm the safety of the three Ebola vaccine regimens, and suggest that an immune response is induced and maintained for up to 12 months.
One of the largest Ebola vaccination trials to date, conducted with both adults and children aged 1 year and older, this vast trial mobilized African, European and US research teams working together in Liberia, Guinea, Sierra Leone, and Mali. In total, the trial included 1400 adults and 1401 children.
In Guinea, the trial was conducted in partnership with the national Ministry of Health, the National Ethics Committee for Health Research (CNERS), the Maferinya Research Center, the National Department of Pharmacy and Medication, and Inserm. It was implemented by ALIMA teams who ensured the enrollment and monitoring of local volunteers for the trial in Conakry and Maferinya.
Building trust for community participation
One of the main challenges in such clinical trials is succeeding in enrolling volunteers from local communities to participate in the trial, as well as successfully monitoring these participants during the course of the study over the years. This can be particularly difficult in rural, hard-to-reach areas where people are prone to move, or have limited access to medical support or information.
The participant inclusion rate for the vaccine trial in Guinea was particularly high, and was a key factor linked to the successful implementation of the PREVAC study. This high participant inclusion and retention rate was largely due to ALIMA’s strong ties with the local community in Guinea, and its experience in community engagement.
ALIMA’s relationship of trust with the local community, created in part due to our expertise responding to several local Ebola outbreaks in the country, meant that we were able to enroll a large number of local participants for the trial, and continue monitoring them as well. ALIMA teams visited villages across the region, and trained over 60 community facilitators to raise awareness on the initiative through surveys, focus groups, and community meetings.
“ALIMA has been in Guinea since 2014, and has responded to several Ebola outbreaks over the years. So not only do we have a unique expertise on Ebola here, but crucially, we have gained the confidence of the people. This was not easy, as Ebola is often stigmatized in the community, and it has taken quite some time to raise awareness and build trust with people,” says Sory Keita, ALIMA Head of Mission in Guinea.
Read the complete press release from the INSERM here.
Read more about ALIMA’s work in Guinea here.